Hospital researchers roll out plan to test thousands of NC patients for COVID symptoms, immunity
Hundreds of thousands of North Carolina patients could soon help researchers at two hospital networks track COVID-19 cases in real-time in an effort partly funded by state legislative leaders.
Posted — UpdatedSome Republican officials across the state – and across the country – have expressed growing skepticism about stay-at-home orders and other measures to combat the spread of COVID-19 as effects of the shutdown continue to ripple through the economy.
Berger's office for weeks has argued for random sampling of the state's population to better inform choices about how long to extend that shutdown. Monday's announcement criticized recent modeling on the issue as "built on guesses."
"This study may also show that the situation is much better than the models project. In that case, I think we can be confident that a reopening of the economy can be done safely," Berger said Wednesday.
Shortages in testing supplies and protective equipment, public health experts have said, have so far made random sampling untenable.
Until the announcement Monday, North Carolina's lead agency on the state's coronavirus response said it was in the dark about the hospital study.
"Though legislative leaders took this action with Wake Forest Baptist Health without coordinating with state health leaders, the Department of Health and Human Services has reached out to scientists performing these tests to learn their research methodology and see how it can supplement current state testing and surveillance efforts," DHHS spokeswoman Kelly Haight Connor said in an email Tuesday.
The research is not quite the random sampling Berger's been pushing for.
Instead, says lead researcher Dr. John Sanders, it's a multi-part plan to gather near real-time data from 750,000 patients of the Wake Forest Baptist Health and Atrium Health hospital networks.
Pulling that off, Sanders said, relies on a lot of volunteers.
"We have to have citizens step up and help us in tracking the disease, following how we’re doing as the disease evolves and as public policy decisions are made that might impact rates one way or another," said Sanders, Wake Forest Baptist's chief of infectious diseases.
Deploying 'syndromic surveillance'
The vast majority of the study's participants will help track the disease by reporting their symptoms through an online portal created by California-based tech giant Oracle – a technique known as syndromic surveillance.
"It's really quick data entry," Sanders said. "It's basically a couple of questions each day."
The portal is designed to securely store patient information and handle hundreds of millions of data entries a day, he said. The platform also allows doctors to link these symptoms with lab results – if a patient eventually tests positive for COVID-19.
"This is not groundbreakingly clever," Sanders said. "This is applying basic epidemiologic techniques, taking advantage of the existing health systems to be able to apply those techniques and get this sort of data."
For now, patients sign up directly through the hospitals, though Sanders said the team is working on ways for other volunteers to enroll.
A separate component of the study – on a smaller subset of patients – will focus on testing for antibodies, signs a person already had COVID-19 and could be immune.
That's where the funding from the legislature's discretionary fund comes in.
The $100,000 contribution is paying for more than 1,000 home-sampling and test kits from two different companies, which started going out to volunteers from the early stage of the study this week.
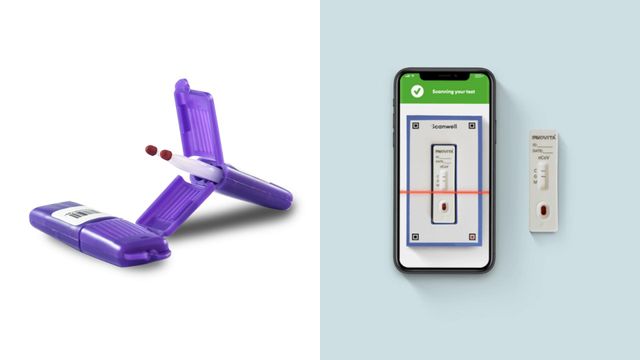
Several hundred of the kits come from the California-based Neoteryx, which manufactures blood collection products about the size of a cotton swab. Patients will use the kits to gather a blood sample from a finger prick and send it back to researchers for analysis.
On Monday night, Los Angeles-based Scanwell Health began shipping out 1,000 of its own kits for the Wake Forest Baptist study. The kits use blood from a finger prick to show the presence of COVID-19 antibodies within minutes. Results, shown on a device similar to a home pregnancy test, can then be uploaded to the patient portal.
Scanwell's devices aren't approved by the Food and Drug Administration for commercial use, and a spokeperson with the agency said Monday that regulators haven't yet granted any companies emergency approval to sell at-home tests for COVID-19.
But the test kits are cleared for clinical research, Sanders said, and his study is one of the efforts aimed at validating their results.
By the end of the month, researchers are planning to send out a mixture of the test kits to 25,000 patients between Wake Forest Baptist and Atrium, a part of the overall study that should provide valuable data on who's already had the disease.
"We know lots of people have experienced symptoms over the last couple of months where they weren't necessarily able to get tested and are concerned that maybe they had COVID," Sanders said.
In this stage, he said, the study is selecting a pool of participants based on demographics and other information. That makes their approach different from true random sampling.
"With random sampling, you need to make sure you have broad enough coverage to make sure your results reflect the population. It's a wonderful way to do sampling if you've got enough tests," he said. "We're not sure we have enough tests to do a purely random sampling of the population, so we're doing a systematic sampling where we're making sure we've got representation"
The team initially planned to send patients tests every month for year, but they may end up scaling back based on costs and logistics.
Sanders acknowledged that it's still unclear whether having antibodies to the virus means a person is safe from it.
"I have repeatedly seen discussion of, when the antibody tests are available, we’ll know who’s immune and who can go right back to work and do whatever," he said. "Frankly, we don’t know that to be the case. We don’t know exactly what the implication of having the antibodies is, which is why we need studies like this."
Data expected in a month
Although a much smaller part than the larger surveillance effort, Sanders said the legislature's funding of the antibody research will allow the research team to start sharing data with larger grant-makers like the Centers for Disease Control and Prevention and the NIH.
"We are outrageously grateful to the state legislature for helping us to get some quick funding to be moving," he said.
As of now though, it's unclear where future funding will come from.
A spokesman for Berger's office said Monday that legislative leaders hadn't ruled out additional contributions.
Sanders said he's hoping to get "first blush data" on symptoms and antibody in a month or so, although that depends on how quickly they're able to sign patients up. The more people enroll, the better the data will be, he said, and the hospitals are actively reaching out to patients now.
The goal is to feed all of this data back to local, state and federal agencies and give policymakers additional information for making public health decisions.
Sanders adds that data on the regional level, specific to North Carolina, is what national health leaders have been asking for in President Donald Trump's daily briefings.
"There's lot's of reasons that we are different regionally," he said. "But straight from Tony Fauci and Debbie Birx every night is the call for better data, defining what the local transmission dynamics are."
• Credits
Copyright 2024 by Capitol Broadcasting Company. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.