FDA committee recommends approval of Pfizer COVID-19 vaccine
Once the FDA approves the Pfizer-BioNTech vaccine for emergency use authorization, Duke Hospital is among the North Carolina facilities that will receive the first doses.
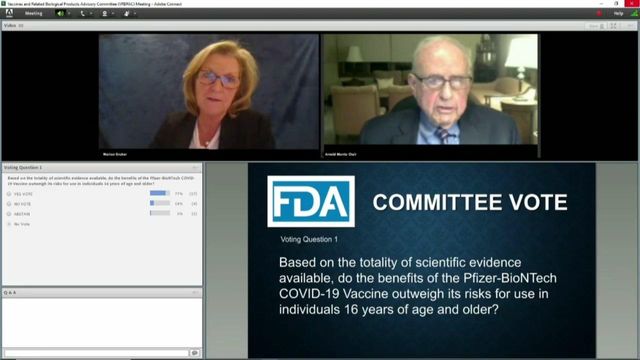
Posted — UpdatedThe vote capped an day-long meeting where FDA regulators interviewed Pfizer executives, as well as experts at the U.S. Centers for Disease Control and Prevention.
In a 17-4 vote, the committee decided the benefits of the vaccine outweigh the potential risks to people aged 16 and older. Prior to the vote, some committee members sought to exclude 16-and17-year olds from the recommendation, citing a lack of data for that age group.
Committee members urged vaccine distributors to be hyper-transparent about potential side effects of the vaccine they're hoping to distribute across the United States.
Responding to questions from committee members, Pfizer Senior Vice Presidents Kathrin Jansen and Bill Gruber said they're monitoring the situation. They said Pfizer trials showed no signals that the vaccine could trigger severe allergic reactions.
They pointed to data showing the Pfizer vaccine has an efficacy rate of 95%, and said more than 43,400 people participated in the trial without suffering any major safety issues.
A committee member said he didn't think the two UK cases were serious enough to halt the approval process. However, committee chairman Arnold Monto noted that some people will wait for updates on the UK cases before agreeing to take the vaccine.
“Facts may be important but perception drives a lot of decisions," Monto said.
FDA committee members on multiple occasions emphasized the importance of sharing information with the American public.
During a public hearing, multiple health experts spoke in favor of approving the vaccine. At the same time, they expressed concern over a lack of information for some demographic groups.
Angela Rasmussen, a virologist and research scientist at Columbia University, was one of several speakers to call for more information for how the vaccine affects Black people, Native Americans, pregnant women and the elderly.
Still, some experts praised the drugmakers' development of the vaccine in record time.
"The potential benefits of this vaccine outweigh the identified risks," said Rossi Hassad, a research and statistics professor at Mercy College who has expertise in Epidemiology.
“I agree with the FDA reviewers that there is no evidence of a major safety signal,” said Peter Lurie, a former associate commissioner at the FDA.
Thursday's hearing provided a glimpse into the challenges of distributing the vaccinations. The Pfizer vaccine needs to be kept at ultra-cold temperatures throughout the distribution process, and it can only be ordered in batches of 975 shots or more.
"The vaccine characteristics of the Pfizer program are very different and unique compared to other vaccines we've seen before and they do impact distribution, site storage, as well as administration requirements," said Anita Patel, deputy incident manager for the CDC’s COVID-19 response.
Those factors have posed some concerns for officials in rural parts of the country where large quantities aren't needed, she said. However, she said the federal and state officials are working with local communities and nursing homes to ensure effective distribution.
People older than 65 are most at-risk for complications from the virus.
Roughly one in 130 seniors who have contracted the virus have need hospitalization, a rate four times higher than the 18-to-49-year-old age group, Dr. Aron Hall, acting chief of the CDC’s respiratory viruses branch, told the committee.
Once the vaccine arrives at Duke, it will be stored properly and vaccination will begin immediately. Duke nurse Frank DeMarco said it's possible people will be vaccinated by Christmas.
"If we are really, seriously going to break that back of this pandemic, this is the way to do it," DeMarco said. "So, I ask people to understand that. Before you make a decision, make an educated decision by talking to your primary care physicians."
The number of COVID-19 infections in the United States could be two to seven times higher than reported to health authorities, Hall, the CDC official, told the FDA on Thursday.
“One of every 2.5 hospitalized cases and one out of every 7.1 non-hospitalized cases may have been nationally reported," Hall said.
DeMarco understands some people will have reservations but said many patients have taken part in the trials and doctors are confident in the vaccine and know from the studies it’s extremely effective.
"People are going to be reluctant, people are going to be afraid or nervous about taking vaccine, that’s understandable," he said. "We have confidence in the studies and trials. We know it’s extremely effective."
Those who work with and near COVID-19 patients and staff and residents of long-term care facilities are at the top of the state’s priority list for vaccines, so it could be months before the general public is able to get vaccinated.
A spokesperson for WakeMed said the hospital system will receive the COVID-19 vaccine soon after Duke and the other priority hospitals.
"WakeMed will receive the COVID-19 vaccine within days of the first 11 healthcare providers," officials explained.
Related Topics
• Credits
Copyright 2024 by Capitol Broadcasting Company. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.