Pompe survivors mark success of drug developed at Duke
Families impacted by an often-deadly, inherited childhood disease have something to celebrate this weekend at Duke's Children's Hospital. It was there that a select group of children with Pompe disease began a drug trial in 2004, and it is there that they reunited in 2013 to celebrate the trial's success.
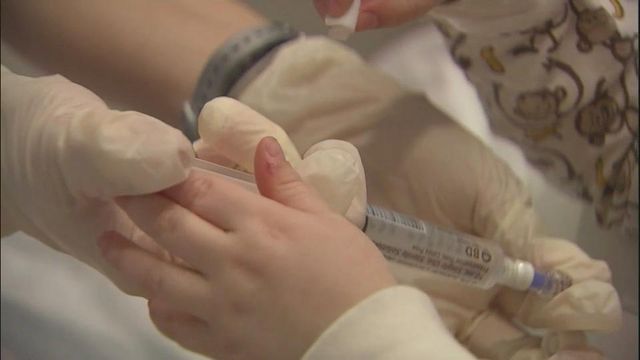
Posted — UpdatedMyozyme was developed at Duke and tested on Pompe patients at the Durham medical center. The drug replaces a key enzyme in the blood to help convert a starch called glycogen into sugar. Without it, glycogen builds up in skeletal muscles and in the heart, damaging muscles and nerves of Pompe patients.
"This disease is so rapidly progressive. Think about a condition where death occurs by about a year of age," said Dr. Priya Kishnani, a pediatric geneticist involved in the trial.
Yamila Jimenez of Peru celebrated her first birthday at Duke in 2004. It was a milestone, because she had been diagnosed with Pompe disease. She and several other children in early clinical trials enjoyed the promise of a longer life due to the drug.
Another member of the trial group, Abdurrahman Vahad of South Africa, has reached the ripe old age of 12, and he still receives the intravenous infusion every two weeks. He probably will for the rest of his life, his father, Ebrahim Vahad said.
"Until something new comes up," he said, "So we'll be looking to Duke to come up with something new."
Abdurrahman and several others will have medical evaluations and a reunion at Duke this week. He especially wanted to see Jason Kelly, now 14 years old.
"He's sort of the yardstick by which we measure Abdurrahman, because he's the oldest," said Rashida Vahad, Abdurrahman's mother.
For parents, families and Duke medical staff, both boys are living miracles. "God makes miracles happen," Rashida Vahad said.
"I think if it were any other people, some of them would have just given up on the first try, and they really pushed through for me, and I'm really thankful because of that," Abdurrahman said.
The FDA has approved and marketed Myozyme, and now it is proving effective in patients with the adult onset form of Pompe disease.
The patients and their families, along with doctors, staff and drug company representatives involved in the Duke program, will gather at a series of events through the weekend.
• Credits
Copyright 2024 by Capitol Broadcasting Company. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.